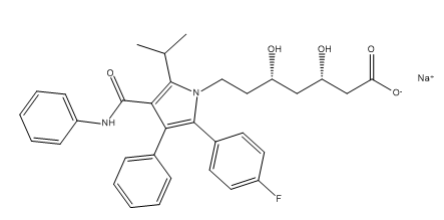
Atorvastatin EP Impurity E Sodium salt
| Product Name | Atorvastatin EP Impurity E Sodium salt |
|---|---|
| Alternate Names | Atorvastatin Impurities, Impurities of Atorvastatin |
| CAT No. | CS-O-07289 |
| CAS No. | 501121-34-2 (Free Acid) |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 580.62 g/mol |
| Mol. For. | C₃₃H₃₄FN₂NaO₅ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Atorvastatin |
| Therapeutic | Anti-Diabetic |
| Smileys | O=C(C(C(C1=CC=CC=C1)=C(C2=CC=C(F)C=C2)N3CC[C@H](O)C[C@H](O)CC([O-])=O)=C3C(C)C)NC4=CC=CC=C4.[Na+] |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Atorvastatin EP Impurity E, also known as Methyl 4-(4-fluorophenyl)-6-isopropyl-2-[(N-methylmethanesulfonamido)methyl]pyridine-3-carboxylate, is a chemical compound that is commonly used in the pharmaceutical industry as a reference standard for quality control purposes. This impurity is typically found in small amounts in Atorvastatin, which is a popular drug used to lower cholesterol levels in patients with hypercholesterolemia.
Atorvastatin EP Impurity E is a pyridine-based compound that contains a fluorophenyl group, a methyl group, and an isopropyl group. The compound has a molecular formula of C22H28FN2O4S and a molecular weight of 442.54 g/mol. It is a white to off-white powder that is sparingly soluble in water but highly soluble in organic solvents like methanol, ethanol, and acetone.
In pharmaceutical applications, Atorvastatin EP Impurity E is used as a reference standard in the development and validation of analytical methods for the quantification of impurities in Atorvastatin. It is also used in the identification and characterization of impurities that may be present in Atorvastatin samples. The use of reference standards like Atorvastatin EP Impurity E ensures that pharmaceutical products are of consistent quality and purity, which is essential for patient safety and efficacy.
Get an Instant Quote
Related Compounds
Difluoro Atorvastatin Acetonide tert-Butyl Ester | Atorvastatin Cyclic (Fluorophenyl) Impurity | Atorvastatin Diepoxide Impurity | Atorvastatin Calcium Propylene Glycol Solvate | Atorvastatin EP Impurity A (Sodium Salt) | Atorvastatin epoxy pyrrolooxazin 7-hydroxy analog | Atorvastatin Acetonide Methyl Ester | Atorvastatin Lactam Sodium Salt Impurity | Atorvastatin Diepoxide Lactone | Atorvastatin EP Impurity C Sodium salt | Atorvastatin Impurity 19 | Atorvastatin EP Impurity B Calcium salt | Atorvastatin Allyl Ester | Atorvastatin Acid Sodium salt | Atorvastatin EP Impurity C | Atorvastatin Cyclic (Fluorophenyl) Sodium | Atorvastatin Isopropyl Ester | Atorvastatin diol Methyl ester impurity | Atorvastatin Lactone Diepoxide | Atorvastatin Dehydro Acid Sodium Salt | Atorvastatin Epoxy Pyrrolooxazin tricyclic Calcium Salt | Atorvastatin Pyrrolidone Analog | Atorvastatin Diepoxide Calcium Salt | Atorvastatin Related compound B | Atorvastatin EP Impurity F (Ca Salt) | Atorvastatin Pyrrolidone Phenanthrene Sodium | Atorvastatin EP Impurity D | Atorvastatin 2-Fluoro Analog | Atorvastatin EP Impurity A (Sodium salt) | Atorvastatin EP Impurity G (3-O-methyl Atorvastatin calcium salt) | Atorvastatin EP Impurity J (Calcium salt) | Atorvastatin Lactone 3-O-Methyl Ether | Atorvastatin Ethyl Ester | ATV-XI | Atorvastatin Acid t-Butyl Ester | Atorvastatin EP Impurity A | Atorvastatin-ATN-2 triamino impurity | Atorvastatin Diketoene Impurity | Atorvastatin Impurity 25 | Atorvastatin Di-acetonide tert-Butyl Ester | Atorvastatin Impurity D | Atorvastatin Amide | Atorvastatin 5-O-Methyl Sodium | N-Nitroso Atorvastatin Impurity | Atorvastatin 3-Deoxy-Hept-2-Enoic Acid Ethyl Ester | Dihydroxy Diketo Atorvastatin Impurity | Difluoro Atorvastatin | Atorvastatin Diamino impurity | Atorvastatin Impurity F | Atorvastatin EP Impurity P calcium Salt | Atorvastatin Impurity 21 | Atorvastatin Pyrrolidone Impurity | Atorvastatin Pyrrolidone Phenanthrene Calcium | Atorvastatin EP Impurity P (Sodium salt) | Atorvastatin 3-Fluoro Analog | Atorvastatin EP Impurity Q sodium salt | sodium (3R,5R)-7-(2-(4-fluorophenyl)-5-isopropyl-3-phenyl-1H-pyrrol-1-yl)-3,5-dihydroxyheptanoate | Allyl Ester of Atorvastatin Cyclic (Isopropyl) Impurity | Atorvastatin Acetonide | Atorvastatin EP Impurity I | Atorvastatin Epoxy Pyrrolooxazin Analog | Atorvastatin Dehydro Sodium Salt (E/Z mixture) | Atorvastatin 3-Deoxyhept-2Z-Enoic Acid Sodium Salt | Atorvastatin EP Impurity B | Atorvastatin Tri-acetonide tert-Butyl Ester | Atorvastatin Impurity 1 | Atorvastatin EP Impurity G Sodium Salt | Atorvastatin Epoxy Tetrahydrofuran Analog | Atorvastatin Lactam Calcium Salt Impurity | Atorvastatin Impurity 17 |