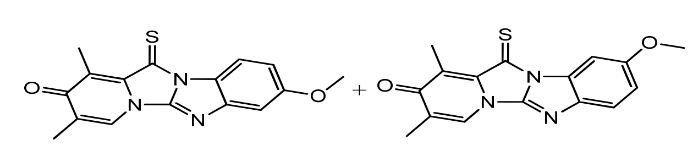
Omeprazole EP impurity F & G Mixture
| Product Name | Omeprazole EP impurity F & G Mixture |
|---|---|
| Alternate Names | Omeprazole Impurities, Impurities of Omeprazole |
| CAT No. | CS-T-57691 |
| CAS No. | 125656-82-8+125656-83-9 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 311.36+311.36 g/mol |
| Mol. For. | C₁₆H₁₃N₃O₂S+C₁₆H₁₃N₃O₂S |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Omeprazole |
| Therapeutic | Anti ulcer |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Omeprazole is a proton-pump inhibitor widely used in the treatment of gastroesophageal reflux disease (GERD), peptic ulcer disease (PUD), and other related disorders. However, during the manufacturing process, impurities may be formed, which can affect the safety and efficacy of the final product. Omeprazole EP Impurity F & G Mixture is one such impurity that is commonly found in Omeprazole formulations.
Omeprazole EP Impurity F & G Mixture is a combination of two impurities, namely 5-methoxy-2-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methylsulfinyl]-1H-benzimidazole (Impurity F) and 2-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methylsulfinyl]-5-(1H-1,2,4-triazol-1-yl) -1H-benzimidazole (Impurity G). These impurities are formed during the manufacturing process due to the reaction of Omeprazole with other chemicals.
The usage of Omeprazole EP Impurity F & G Mixture is primarily for research and development purposes. It is used as a reference standard to identify and quantify the presence of these impurities in Omeprazole formulations. The chemical information of Omeprazole EP Impurity F & G Mixture includes its molecular weight, molecular formula, and structural formula.
In conclusion, Omeprazole EP Impurity F & G Mixture is an important reference material used by researchers and manufacturers to ensure the quality and safety of Omeprazole formulations.
Get an Instant Quote
Related Compounds
Omeprazole Impurity 53 | Omeprazole Impurity- K | Desulfoxide 4-Demethyl Omeprazole | Omeprazole Impurity 30 | Omeprazole Impurity 24 | Omeprazole Impurity 79 | Omeprazole Impurity 25 | Omeprazole EP Impurity A | Omeprazole impurity D | Omeprazole Related Compound 5 | Omeprazole Acid Methyl Ester Sulfide | Omeprazole Impurity 8 | O,O-Didesmethyl Omeprazole | Omeprazole EP Impurity C | Omeprazole EP Impurity G | Omeprazole impurity (DIMER Mixture) | Omeprazole O-hydrogen sulfite | Omeprazole Impurity 60 | Bis-Desmethoxy Omeprazole Sulfide | o-Toluoyl-5-hydroxy Omeprazole Sulfide | Omeprazole Related Compound 7 | N-Nitroso Omeprazole EP Impurity I | N-methyl Omeprazole | Omeprazole D15 | N-Methyl Esomeprazole Isomer-1 | Omeprazole dibenzimidazole Impurity | Omeprazole D13 | Omeprazole Hydrolysis Impurity | Omeprazole Impurity 63 | Omeprazole-N-(S)-camphorsulfonamide | N-Nitroso Omeprazole Sulfide | N-Nitroso Omeprazole sodium | Omeprazole Isomer-2 5-Methoxy -1-[(4-methoxy-3,5-dimethyl-2-pyridinyl)]-0[2-(4-methoxy-3,5-dimethyl -2-pyridinyl)methyl]-sulfanyl]-1H-banzimidole | Omeprazole EP Impurity F | Omeprazole sulfide 5-carboxylic acid | Omeprazole EP impurity I | Omeprazole EP impurity B | Omeprazole Sulfide N1-Methyl 5-Methoxy Analog | N-Methyl Omeprazole (Mixture of isomers with the methylated nitrogens of imidazole) | N-(4-Methoxy-3,5-dimethyl-2-pyridinyl)methyl Omeprazole |