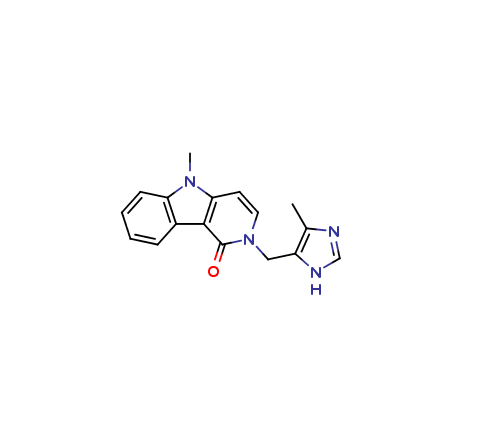
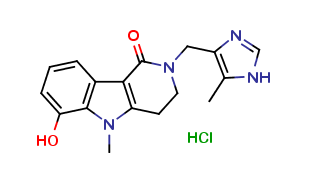
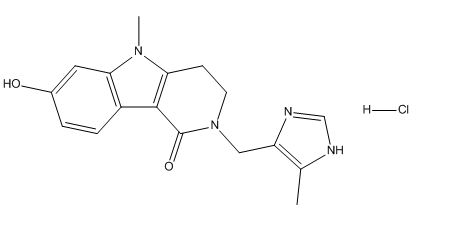
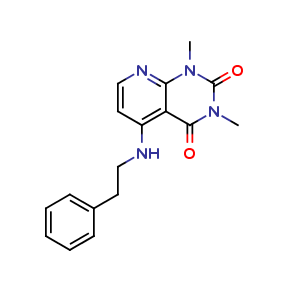
Alosetron Impurities and its Related Products
Alosetron is a medication used to treat irritable bowel syndrome (IBS) in women. However, during its manufacturing process, impurities may be formed which can have adverse effects on human health. These impurities can include related compounds of Alosetron or even by-products of the manufacturing process. It is important to monitor and control the levels of these impurities to ensure the safety and efficacy of the medication.