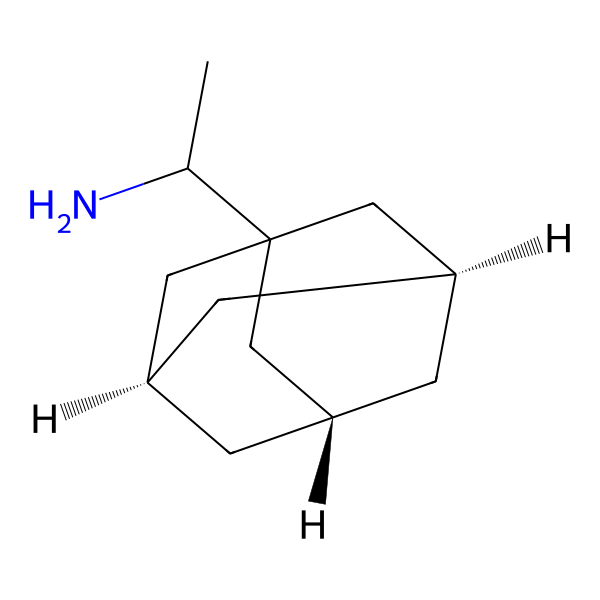
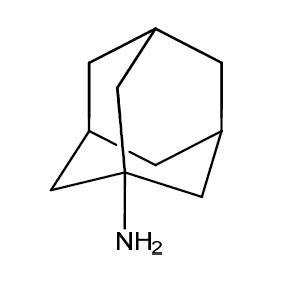
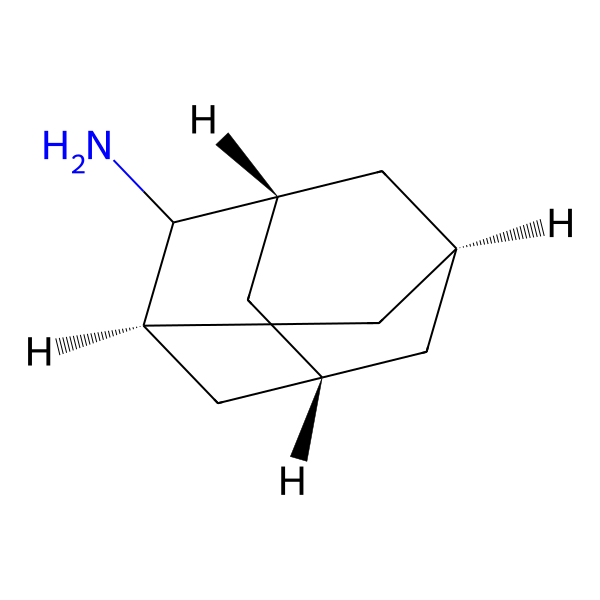
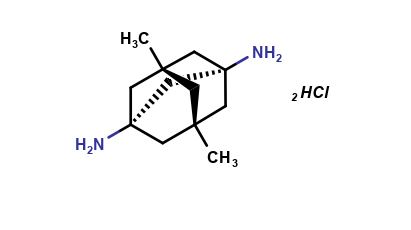
Amantadine Impurities and its Related Products
Amantadine is an antiviral drug used for the treatment and prevention of influenza A. Impurities are unwanted substances that may be present in the drug product during manufacturing or storage. These impurities can affect the quality, safety, and efficacy of the drug product. The identification and control of impurities are important for the quality assurance of the drug product. The most common impurities found in Amantadine include related compounds, residual solvents, and degradation products. The presence of these impurities should be monitored and controlled to ensure the safety and efficacy of the drug product.