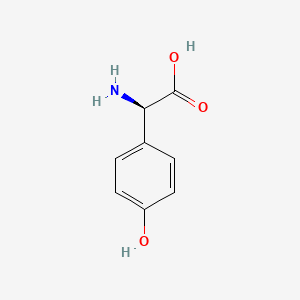
Amoxicillin EP Impurity I
| Product Name | Amoxicillin EP Impurity I |
|---|---|
| Alternate Names | Amoxicillin Impurities, Impurities of Amoxicillin |
| CAT No. | CS-O-07195 |
| CAS No. | 22818-40-2 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 167.16 g/mol |
| Mol. For. | C8H9NO3 |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Amoxicillin |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Amoxicillin EP Impurity I is a chemical compound that is commonly used in the pharmaceutical industry as a reference standard in quality control testing of Amoxicillin drug formulations. It is also used in research and development of new drugs, and in the development of analytical methods for the detection and quantification of Amoxicillin and its related compounds.
Chemically, Amoxicillin EP Impurity I is a degradation product of Amoxicillin, which is an antibiotic in the penicillin group of drugs. It is a white to off-white powder that is soluble in water and ethanol. The chemical name of Amoxicillin EP Impurity I is (2S,5R,6R)-6-{[(2R)-2-Amino-2-(4-hydroxyphenyl)acetyl]amino}-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid.
Amoxicillin EP Impurity I is used as a reference standard in the analysis of Amoxicillin drug formulations for purity, stability, and quality. It is also used in the development of analytical methods for the detection and quantification of Amoxicillin and its related compounds. The use of this reference standard ensures the accuracy and reliability of the analytical results obtained during quality control testing.
In conclusion, Amoxicillin EP Impurity I is a vital chemical compound in the pharmaceutical industry, and its usage in quality control testing of Amoxicillin drug formulations plays a significant role in ensuring the safety and efficacy of the drug.
Get an Instant Quote
Related Compounds
Amoxicillin Open RIng Trimer Impurity sodium salt | Amoxicillin Related Compound M Sodum salt | Amoxicillin EP Impurity K trisodium salt | Amoxicillin Impurity M | Amoxicillin Related Compound M | Amoxicillin Trihydrate - Impurity K | Sterile Clavulanate Potassium+Amoxicillin | Amoxicillin EP Impurity L | Amoxicillin Related Compound C | Amoxicillin Related Compound E | Amoxicillin EP Impurity J | Amoxicillin EP Impurity C | Amoxicillin EP Impurity H | Amoxicillin Close ring Trimer | Amoxicillin Open Ring Trimer Impurity | Amoxicillin Open Ring Decarboxylated Dimer | Amoxicillin oxide | Amoxicillin EP Impurity B | Amoxicilline EP Impurity D (Mixture of isomers) | Amoxicillin Trihydrate - Impurity J | Amoxicillin EP Impurity P | Amoxicillin EP Impurity H | N-Pivaloyl Amoxicillin | N-(Hydroxyphenylglycyl) amoxicillin Sodium salt | Amoxicillin Trimer | Amoxicillin double Side Chain Impurity | Amoxicillin EP Impurity K | Amoxicillin Dimer (Penicilloic acid form) | Amoxicillin Impurity C (R Isomer) | Amoxicillin Trimer Trisodium Salt | N-Nitroso Amoxicillin | Amoxicilloic Acid | rac-Amoxicillin EP Impurity H | p-hydroxyphenylglycine methyl ester | Amoxicillin EP Impurity F | Amoxicillin Related Compound D | Amoxicillin Open Ring Ethyl | Amoxicillin Aldehyde | Amoxicillin EP Impurity O | Amoxicillin EP Impurity J Disodium salt |