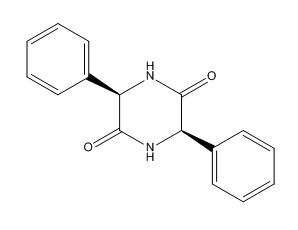
Ampicillin EP Impurity G
| Product Name | Ampicillin EP Impurity G |
|---|---|
| Alternate Names | Ampicillin Impurities, Impurities of Ampicillin |
| CAT No. | CS-O-07205 |
| CAS No. | 31485-02-6 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 266.29 g/mol |
| Mol. For. | C₁₆H₁₄N₂O₂ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Ampicillin |
| Therapeutic | Antibiotics |
| Smileys | C1=CC=C(C=C1)C2C(=O)NC(C(=O)N2)C3=CC=CC=C3 |
| Canonical Smiles | C1=CC=C(C=C1)C2C(=O)NC(C(=O)N2)C3=CC=CC=C3 |
| InchIKey | BMGJGWGTAAJISM-ZIAGYGMSSA-N |
| Inchl | InChI=1S/C16H14N2O2/c19-15-13(11-7-3-1-4-8-11)17-16(20)14(18-15)12-9-5-2-6-10-12/h1-10,13-14H,(H,17,20)(H,18,19)/t13-,14-/m1/s1 |
| IUPAC | (3R,6R)-3,6-diphenylpiperazine-2,5-dione |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Ampicillin EP Impurity G is a chemical compound that is used in pharmaceutical research and development as a reference standard for the identification and quantification of impurities present in ampicillin formulations. It is a highly pure and stable compound that is used as a standard for analytical testing methods such as high-performance liquid chromatography (HPLC) and gas chromatography (GC).
Chemically, Ampicillin EP Impurity G is known as 2,6-diaminopimelic acid (DAP) and is a naturally occurring amino acid that is found in the peptidoglycan layer of bacterial cell walls. It is a derivative of lysine and is a key component in the biosynthesis of peptidoglycan, which provides structural support to the bacterial cell wall.
In pharmaceutical research, Ampicillin EP Impurity G is used to assess the purity of ampicillin formulations and to ensure that they meet the quality standards set by regulatory bodies such as the European Pharmacopoeia (EP). It is also used as a reference standard for the development and validation of analytical testing methods used in the quantification of impurities in ampicillin formulations.
Overall, Ampicillin EP Impurity G plays a critical role in ensuring the safety and efficacy of ampicillin formulations, and is an important tool for pharmaceutical researchers and manufacturers in the development of high-quality pharmaceutical products.
Get an Instant Quote
Related Compounds
Ampicillin open ring DImer | Ampicillin EP Impurity D | Ampicillin EP Impurity E | Ampicillin EP Impurity L | Ampicillin Dimer Impurity | Ampicillin EP Impurity J | Ampicillin oligomer 1 (trimer) | Ampicillin EP Impurity I | Pivampicillin Impurity A | Ampicillin EP Impurity K | Ampicillin EP impurity C | Ampicillin Impurity p | Ampicillin EP Impurity I HCl | Ampicillin thiazepine analog | Ampicillin EP Impurity F | Ampicillin EP Impurity B | Ampicillin oligomer 2 | Pivampicillin Impurity B | Ampicillin Oligomer 1 (trimer) Sodium Salt | D-Phenylglycinamide Hydrochloride | N-Piperacillinyl Ampicillin | Ampicillin Impurity O | Ampicillin EP Impurity N |