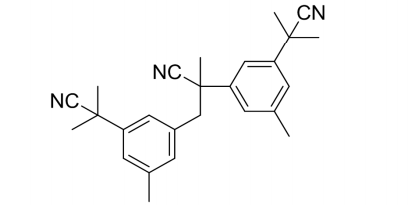
Anastrozole Impurity A
| Product Name | Anastrozole Impurity A |
|---|---|
| Alternate Names | Anastrozole Impurities, Impurities of Anastrozole |
| CAT No. | CS-O-31401 |
| CAS No. | 918312-71-7 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 383.53 g/mol |
| Mol. For. | C26H29N3 |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Anastrozole |
| Purity | 98.47 |
| Therapeutic | Anti-Cancer / Oncology |
| Canonical Smiles | CC1=CC(=CC(=C1)C(C)(C)C#N)CC(C)(C#N)C2=CC(=CC(=C2)C(C)(C)C#N)C |
| InchIKey | DSWIROKRYFYWHD-UHFFFAOYSA-N |
| Inchl | InChI=1S/C26H29N3/c1-18-8-20(12-21(9-18)24(3,4)15-27)14-26(7,17-29)23-11-19(2)10-22(13-23)25(5,6)16-28/h8-13H,14H2,1-7H3 |
| IUPAC | 2,3-bis[3-(2-cyanopropan-2-yl)-5-methylphenyl]-2-methylpropanenitrile |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Anastrozole Impurity A (also known as 4,4'-Difluorobenzophenone) is a synthetic organic compound that is commonly used as an impurity standard in the manufacturing of Anastrozole, a medication used to treat breast cancer in postmenopausal women. This impurity is typically found in small amounts in Anastrozole, but it is important to monitor its levels due to potential adverse effects on the patient's health.
Chemically, Anastrozole Impurity A is a derivative of benzophenone, which is a common organic building block used in the synthesis of various pharmaceuticals and industrial chemicals. It is a crystalline solid with a molecular weight of 236.19 g/mol and a melting point of 63-65°C.
In terms of usage, Anastrozole Impurity A is primarily used as a reference standard in analytical testing to ensure the purity and quality of Anastrozole. It is also used in the development and validation of analytical methods for the quantitative determination of Anastrozole in various matrices such as blood, plasma, and urine.
Overall, Anastrozole Impurity A plays a critical role in the quality control of Anastrozole and helps ensure the safety and efficacy of this important medication in the treatment of breast cancer. Its chemical properties and usage make it an essential component in the pharmaceutical industry.
Get an Instant Quote
Related Compounds
Di-destriazole Desmethyl Anastrozole Dimer Impurity | Anastrozole EP Impurity E | Anastrozole Isomer | Anastrozole diamide | Anastrozole Mono Acid | Anastrozole EP Impurity C | Anastrozole Monoacid monoamide | Hydroxy Anastrozole | Anastrozole EP Impurity H | Anastrozole Monoamide Mononitrile | Anastrozole USP related Impurity e | Anastrozole EP Impurity A | Anastrozole EP Impurity I |