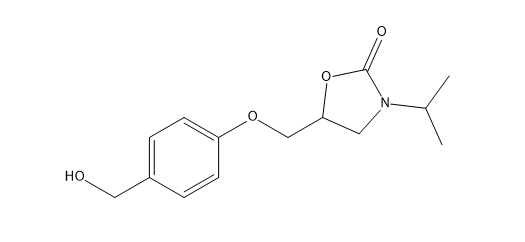
Bisoprolol EP Impurity U
| Product Name | Bisoprolol EP Impurity U |
|---|---|
| CAT No. | CS-P-07246 |
| CAS No. | 1071765-44-0 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 265.31 g/mol |
| Mol. For. | C₁₄H₁₉NO₄ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Therapeutic | Anti-Hypertensives |
| Canonical Smiles | CC(C)N1CC(OC1=O)COC2=CC=C(C=C2)CO |
| InchIKey | PDLSQIVAMWBZTI-UHFFFAOYSA-N |
| Inchl | InChI=1S/C14H19NO4/c1-10(2)15-7-13(19-14(15)17)9-18-12-5-3-11(8-16)4-6-12/h3-6,10,13,16H,7-9H2,1-2H3 |
| IUPAC | 5-[[4-(hydroxymethyl)phenoxy]methyl]-3-propan-2-yl-1,3-oxazolidin-2-one |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Bisoprolol EP Impurity U is a synthetic compound that is used in the pharmaceutical industry as a reference standard and impurity in the analysis of Bisoprolol, a beta-blocker medication used to treat hypertension and other cardiovascular conditions. Bisoprolol EP Impurity U is a structurally related compound that is chemically known as 1-(4-methoxyphenyl)-2-(1-methylethylamino)ethanone.
Bisoprolol EP Impurity U is typically used in the quality control and analysis of Bisoprolol products to ensure that they meet strict regulatory standards for purity and potency. The compound is often used as a reference standard in analytical assays, such as high-performance liquid chromatography (HPLC), to identify and quantify impurities in Bisoprolol formulations.
Chemically, Bisoprolol EP Impurity U is a white to off-white crystalline powder that is soluble in organic solvents such as methanol and acetonitrile. It has a molecular weight of 179.26 g/mol and a melting point of 72-75°C. The compound is considered stable under normal storage conditions and is typically stored in a cool, dry place away from light and moisture.
In summary, Bisoprolol EP Impurity U is an important reference standard and impurity in the pharmaceutical industry that is used to ensure the quality and purity of Bisoprolol products. Its chemical properties and usage make it an essential tool for pharmaceutical companies and regulatory agencies in the development and analysis of cardiovascular medications.
Get an Instant Quote
This page contains information about Bisoprolol EP Impurity U. You can buy Bisoprolol EP Impurity U from Clearsynth at best competitive price with assured price guarantee. Clearsynth offers best quality Bisoprolol EP Impurity U