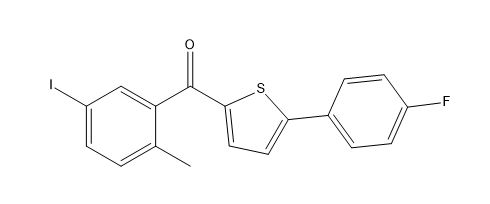
Canagliflozin impurity F
| Product Name | Canagliflozin impurity F |
|---|---|
| Alternate Names | Canagliflozin Impurities, Impurities of Canagliflozin |
| CAT No. | CS-AQ-00195 |
| CAS No. | 1071929-08-2 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 422.26 g/mol |
| Mol. For. | C₁₈H₁₂FIOS |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Canagliflozin |
| Purity | >98% |
| Therapeutic | Anti-Diabetic |
| Smileys | O=C(C(C=C(I)C=C1)=C1C)C2=CC=C(C3=CC=C(F)C=C3)S2 |
| Canonical Smiles | CC1=C(C=C(C=C1)I)C(=O)C2=CC=C(S2)C3=CC=C(C=C3)F |
| InchIKey | UCEUYWYTFYVWBY-UHFFFAOYSA-N |
| Inchl | InChI=1S/C18H12FIOS/c1-11-2-7-14(20)10-15(11)18(21)17-9-8-16(22-17)12-3-5-13(19)6-4-12/h2-10H,1H3 |
| IUPAC | [5-(4-fluorophenyl)thiophen-2-yl]-(5-iodo-2-methylphenyl)methanone |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Canagliflozin impurity F is a chemical compound that is commonly used in the pharmaceutical industry as an impurity in the production of Canagliflozin, which is a drug used to treat type 2 diabetes. Canagliflozin impurity F is also known as (2S,3R,4S,5S,6R)-2-(4-chloro-3-(4-ethoxybenzyl)phenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol.
The chemical formula of Canagliflozin impurity F is C21H25ClO7 and it has a molecular weight of 436.88 g/mol. It is a white to off-white powder with a melting point of 132-136°C. This impurity is known to be a potential genotoxic impurity and thus, its presence in the final drug product should be less than the acceptable limits set by regulatory bodies.
Canagliflozin impurity F is used in the production of Canagliflozin as a starting material or intermediate. The impurity is introduced during the synthesis process and is subsequently removed through various purification steps to obtain the final drug product.
In conclusion, Canagliflozin impurity F is an important intermediate in the production of Canagliflozin, a drug used to treat type 2 diabetes. Its usage is strictly regulated, and its presence in the final drug product should be minimized.
Get an Instant Quote
Related Compounds
Canagliflozin Open Ring Impurity | Canagliflozin Enantiomer impurity | Canagliflozin Peroxide Impurity | Canagliflozin Impurity B | Canagliflozin-S-Furanose | Monoacetyl Canagliflozin | Canagliflozin 2,3-dehydroxy impurity | Canagliflozin impurity 11 | Canagliflozin Impurity 22 | TMS- Hydroxy Canagliflozin | Canagliflozin R-Furanose | Canagliflozin hydroperoxy impurity | Canagliflozin impurity D | Canagliflozin-10 Lactol | Canagliflozin impurity C | Canagliflozin Impurity 10 | Canagliflozin DI D-glucitol IMPURITY | Canagliflozin 2-Methyl Phenyl Impurity | Tetra Acetyl Canagliflozin | Canagliflozin impurity E | Canagliflozin Hydroxy Impurity | Canagliflozin Impurity 20 | Canagliflozin α Isomer | Canagliflozin Dimer | Canagliflozin DIsulfOXIDE impurity | Canagliflozin impurity 12 | Desfluoro Canagliflozin | Canagliflozin Impurity 23 | Canagliflozin Dimethoxy Impurity | Canagliflozin Dimer-1 |