Ciprofloxacin EP Impurity E
| Product Name | Ciprofloxacin EP Impurity E |
|---|---|
| Alternate Names | Ofloxacin Impurities, Impurities of Ofloxacin |
| CAT No. | CS-O-07645 |
| CAS No. | 105394-83-0 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 287.33 g/mol |
| Mol. For. | C₁₆H₁₈FN₃O |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Ofloxacin |
| Purity | 95% |
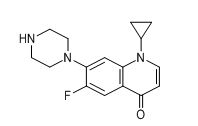
| Smileys | O=C1C2=CC(F)=C(N3CCNCC3)C=C2N(C4CC4)C=C1 |
| Canonical Smiles | C1CC1N2C=CC(=O)C3=CC(=C(C=C32)N4CCNCC4)F |
| InchIKey | QDYUSAJJDOBXBM-UHFFFAOYSA-N |
| Inchl | InChI=1S/C16H18FN3O/c17-13-9-12-14(10-15(13)19-7-4-18-5-8-19)20(11-1-2-11)6-3-16(12)21/h3,6,9-11,18H,1-2,4-5,7-8H2 |
| IUPAC | 1-cyclopropyl-6-fluoro-7-piperazin-1-ylquinolin-4-one |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Ciprofloxacin EP Impurity E is a chemical compound that is used in the pharmaceutical industry as a reference standard. It is a known impurity of the antibiotic drug, Ciprofloxacin, which is used to treat a range of bacterial infections. The impurity is typically used as a reference sample for quality control purposes during the production and testing of Ciprofloxacin.
Chemically, Ciprofloxacin EP Impurity E is known as 1-Cyclopropyl-6-fluoro-4-oxo-7-(1-piperazinyl)-1,4-dihydroquinoline-3-carboxylic acid. It is a white or off-white crystalline powder that is sparingly soluble in water and highly soluble in organic solvents such as methanol and acetonitrile.
In terms of its toxicity, there is limited information available on the specific effects of Ciprofloxacin EP Impurity E on human health. However, as with any chemical substance, it is important to handle it with care and take appropriate safety precautions when working with it.
Overall, Ciprofloxacin EP Impurity E plays an important role in ensuring the quality and safety of Ciprofloxacin as a pharmaceutical product. Its use as a reference standard helps to ensure that Ciprofloxacin is produced to a consistent and high standard, and that it is safe for use in treating bacterial infections.
Get an Instant Quote
Related Compounds
N-Nitroso Ciprofloxacin | Ofloxacin Dihydrooxazole Impurity | N,N’-Desethylene Ofloxacin Hydrochloride | N-Ethoxycarbonyl Ciprofloxacin | Ciprofloxacin EP Impurity B | N-Nitroso Ofloxacin tetrafluorobenzoyl intermediate | Ofloxacin Impurity A | Ciprofloxacin EP Impurity F | Ciprofloxacin Impurity 8 | Ofloxacin N-oxide | Ciprofloxacin Impurity I HCl | Ciprofloxacin Impurity 10 | Ciprofloxacin N-Oxide | Ciprofloxacin EP Impurity E HCl | N-Desmethyl Danofloxacin | N-Nitroso N-Desmethyl Ofloxacin | Ciprofloxacin Methoxy Analog | Desmethyl Dextrofloxacin | Ofloxacin Desmethylpiperazin Methylester | Ciprofloxacin EP Impurity D | Ofloxacin EP impurity E | Ofloxacin EP Impurity D Ethyl Ester | N-Formyl Ciprofloxacin | N-Desmethyl Ofloxacin | Ciprofloxacin Impurity 7 | Ciprofloxacin Impurity 5 | Ciprofloxacin Impurity 4 | Desmethyl Ofloxacin Hydrochloride | Ciprofloxacin Impurity 9 | Ciprofloxacin Impurity 11 |