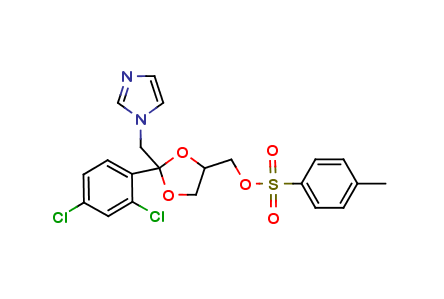
Ketoconazole Impurity E
| Product Name | Ketoconazole Impurity E |
|---|---|
| Alternate Names | Ketoconazole Impurities, Impurities of Ketoconazole |
| CAT No. | CS-P-00437 |
| CAS No. | 134071-44-6 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 483.36 g/mol |
| Mol. For. | C₂₁H₂₀Cl₂N₂O₅S |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Ketoconazole |
| Purity | 95% |
| Therapeutic | Anti-Fungals |
| Smileys | O=S(C1=CC=C(C)C=C1)(OCC2OC(CN3C=CN=C3)(C4=CC=C(Cl)C=C4Cl)OC2)=O |
| Canonical Smiles | CC1=CC=C(C=C1)S(=O)(=O)OCC2COC(O2)(CN3C=CN=C3)C4=C(C=C(C=C4)Cl)Cl |
| InchIKey | WAXNIYHZFWRPGS-UTKZUKDTSA-N |
| Inchl | InChI=1S/C21H20Cl2N2O5S/c1-15-2-5-18(6-3-15)31(26,27)29-12-17-11-28-21(30-17,13-25-9-8-24-14-25)19-7-4-16(22)10-20(19)23/h2-10,14,17H,11-13H2,1H3/t17-,21+/m1/s1 |
| IUPAC | [(2R,4R)-2-(2,4-dichlorophenyl)-2-(imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methyl 4-methylbenzenesulfonate |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Ketoconazole Impurity E is a byproduct that can occur during the synthesis of Ketoconazole, a commonly used antifungal medication. This impurity, also known as 1-Acetyl-4-cyclohexylpiperazine, is a cyclic tertiary amine with a molecular formula of C12H20N2O. It is classified as a heterocyclic compound due to the presence of a nitrogen atom in the piperazine ring.
While Ketoconazole Impurity E is not intended for use in pharmaceutical products, it is important to monitor its levels in Ketoconazole formulations to ensure product quality and safety. Excessive levels of impurities can impact the stability and efficacy of the drug, leading to potential adverse effects in patients.
Chemically, Ketoconazole Impurity E is a relatively stable compound with a melting point of 72-76°C. It is soluble in organic solvents such as methanol, ethanol, and chloroform, but insoluble in water. It is also sensitive to light and should be stored in a cool, dry place away from direct sunlight.
In summary, Ketoconazole Impurity E is a byproduct of the synthesis of Ketoconazole that can impact the quality and safety of pharmaceutical products. Its chemical properties and solubility characteristics make it important to monitor its levels in drug formulations to ensure product efficacy and safety.
Get an Instant Quote
Related Compounds
Ketoconazole Impurity 25 | DextroKetoconazole | N-Acetylpiperazine-N'-(4-phenol) Ketoconazole | Ketoconazole Impurity C | Ketoconazole impurity 1 | N-Nitroso Ketoconazole Impurity D | Ketoconazole Impurity 3 | Cis-tosylate Ketoconazole | Ketoconazole Impurity D | Levoketoconazole | Ketoconazole Impurity 7 | Ketoconazole Hydroxy Impurity |