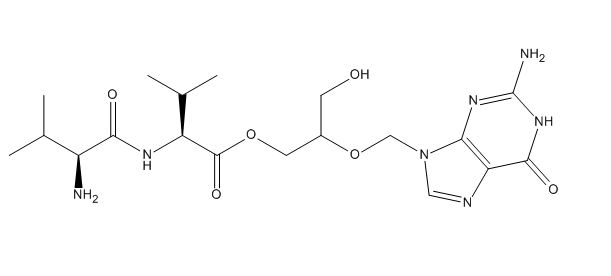
Valganciclovir N-Valyl Impurity
| Product Name | Valganciclovir N-Valyl Impurity |
|---|---|
| Alternate Names | Ganciclovir Impurities, Impurities of Ganciclovir |
| CAT No. | CS-O-16427 |
| CAS No. | 897937-73-4 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 453.49 g/mol |
| Mol. For. | C₁₉H₃₁N₇O₆ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Ganciclovir |
| Purity | >98 % |
| Therapeutic | Antiretroviral / Anti-HIV |
| Smileys | OCC(COC([C@H](C(C)C)NC([C@@H](N)C(C)C)=O)=O)OCN1C(NC(N)=NC2=O)=C2N=C1 |
| Canonical Smiles | CC(C)C(C(=O)NC(C(C)C)C(=O)OCC(CO)OCN1C=NC2=C1N=C(NC2=O)N)N |
| InchIKey | JZCILZBQRWUKME-SPOOISQMSA-N |
| Inchl | InChI=1S/C19H31N7O6/c1-9(2)12(20)16(28)23-13(10(3)4)18(30)31-6-11(5-27)32-8-26-7-22-14-15(26)24-19(21)25-17(14)29/h7,9-13,27H,5-6,8,20H2,1-4H3,(H,23,28)(H3,21,24,25,29)/t11?,12-,13-/m0/s1 |
| IUPAC | [2-[(2-amino-6-oxo-1H-purin-9-yl)methoxy]-3-hydroxypropyl] (2S)-2-[[(2S)-2-amino-3-methylbutanoyl]amino]-3-methylbutanoate |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Valganciclovir N-Valyl Impurity is a chemical compound that is used in the pharmaceutical industry to ensure the purity and quality of the medication Valganciclovir. Valganciclovir is an antiviral medication that is used to treat certain viral infections, such as cytomegalovirus (CMV) retinitis in patients with AIDS. The N-Valyl Impurity of Valganciclovir is a known impurity that is found in small amounts in the medication. It is important to monitor the level of this impurity in the medication to ensure that it is within acceptable limits, as high levels of impurities can affect the effectiveness and safety of the medication.
The chemical formula of Valganciclovir N-Valyl Impurity is C14H22N4O5. It is a white to off-white solid that is soluble in water and methanol. It is classified as an amino acid derivative and is a byproduct of the synthesis of Valganciclovir. The impurity is formed when Valganciclovir is synthesized using a specific process that involves the use of N-Valyl glycine. The impurity is typically present in the medication at a level of less than 0.1%.
Overall, the monitoring and control of Valganciclovir N-Valyl Impurity is essential to ensure the safety and efficacy of the medication.
Get an Instant Quote
Related Compounds
Valganciclovir D-VAline N-(D-Valyl) Impurity | Acetyl Valganciclovir | Valganciclovir dimer (stereoisomer B) | Ganciclovir EP Impurity I | Ganciclovir Mono-O-propionate | Valganciclovir USP RC D (Mixture of Diastereomers) | Valganciclovir Impurity 3 | Valganciclovir Homolog(component G as per USP) | O-Methylganciclovir | Valganciclovir Chloro Diastereoisomer-1 | Valganciclovir N3,N3’-Methylene Dimer | N-Methyl Valganciclovir Hydrochloride (Mixture of Diastereomers) | N-Nitroso Valganciclovir | Valganciclovir HOMOLOGUE 2 | Ganciclovir Diether Impurity | Valganciclovir USP RC D | D-valinate Chlorovalganciclovir | Ganciclovir Related Compound A | Gancyclovir Impurity A | Ganciclovir Bis-Valine Ester Dihydrochloride | N-(L-Valyl) Valganciclovir Hydrochloride |