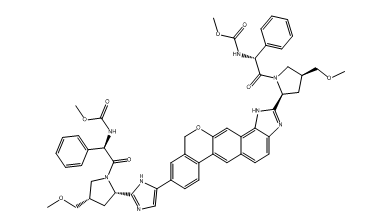
Velpatasvir impurity 1
| Product Name | Velpatasvir impurity 1 |
|---|---|
| Alternate Names | Velpatasvir Impurities, Impurities of Velpatasvir |
| CAT No. | CS-O-15963 |
| CAS No. | 1493769-28-0 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 947.04 g/mol |
| Mol. For. | C₅₃H₅₄N₈O₉ |
| Hazardous | This is not a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Velpatasvir |
| Purity | Not less than 98% |
| Therapeutic | Antiretroviral / Anti-HIV |
| Smileys | O=C(OC)N[C@H](C1=CC=CC=C1)C(N2[C@H](C3=NC=C(C4=CC5=C(C6=CC7=CC=C8C(NC([C@H]9N(C([C@H](NC(OC)=O)C%10=CC=CC=C%10)=O)C[C@@H](COC)C9)=N8)=C7C=C6OC5)C=C4)N3)C[C@H](COC)C2)=O |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Velpatasvir impurity 1 is a chemical compound that is used as a reference standard in the production and testing of Velpatasvir, a medication used in the treatment of chronic hepatitis C virus (HCV) infection. Velpatasvir impurity 1 is a byproduct of the synthesis of Velpatasvir, and its chemical structure is similar to that of the active pharmaceutical ingredient (API). However, Velpatasvir impurity 1 contains an impurity or a chemical component that is not present in the API.
In the pharmaceutical industry, impurities are compounds that are not the intended product but are present in the drug substance or drug product as a result of the manufacturing process. Impurities can affect the safety, efficacy, and quality of the drug product, so it is crucial to identify and control them.
Velpatasvir impurity 1 is typically used as a reference standard in analytical methods such as high-performance liquid chromatography (HPLC) and mass spectrometry (MS) to identify and quantify the impurity in Velpatasvir API and drug products. The impurity level in the API must be below the specified limit to ensure the quality and safety of the drug product.
The chemical name of Velpatasvir impurity 1 is (2S, 3R, 4S, 5S)-2-(2-(6-(cyclopentylamino)-9,9-dimethyl-9,10-dihydroacridin-3-yl)acetamido)-5-((S)-3-(((1S)-1-(methoxymethyl)pyrrolidin-2-yl)methoxy)-1-oxopropan-2-yl)-4-oxo-1,3-oxazolidine-3-carboxylic acid. Its molecular formula is C34H47N5O8, and its molecular weight is 669.78 g/mol.
Get an Instant Quote
Related Compounds
Boc Velpatasvir R, R Isomer (Imidazole and Methoxy Methyl) | Velpatasvir Intermediate-I S,S-Isomer | Des (N-(Methoxycarbonyl)) Velpatasvir | Di Boc Velpatasvir impurity 1 | Boc Velpatasvir Keto Impurity | Velpatasvir copovidone | epi-Velpatasvir | Des (N-(Methoxycarbonyl)-L-valine) Velpatasvir | Di Boc Velpatasvir Keto impurity 1 | Velpatasvir Diastereomer 1 (Velpatasvir R, R Isomer (Imidazole and Methoxy Methyl)) | N-Nitroso Velpatasvir intermediate | Boc Velpatasvir R, R, R Isomer (Imidazole, Methoxy Methyl and Methyl) | Velpatasvir R Isomer (Methoxy Methyl) | Boc Velpatasvir intermediate R, R Isomer (Imidazole and Methoxy Methyl) | VELPATASVIR D6 | Velpatasvir Diastereomer 16 (D-valine Velpatasvir) |