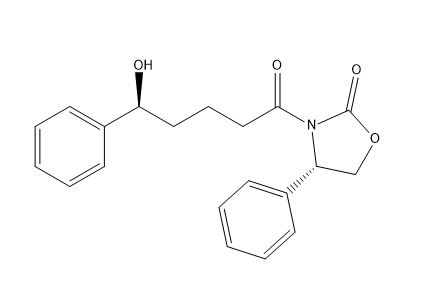
Ezetimibe Dides fluoro impurity
| Product Name | Ezetimibe Dides fluoro impurity |
|---|---|
| Alternate Names | Ezetimibe Impurities, Impurities of Ezetimibe |
| CAT No. | CS-O-15762 |
| CAS No. | Not Available |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 339.39 g/mol |
| Mol. For. | C₂₀H₂₁NO₄ |
| Hazardous | This is not a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Ezetimibe |
| Therapeutic | Anti-Hyperlipidemics |
| Smileys | O[C@H](C1=CC=CC=C1)CCCC(N2[C@@H](C3=CC=CC=C3)COC2=O)=O |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Ezetimibe Dides fluoro impurity is a chemical compound that is commonly used in the manufacturing of pharmaceutical products. This compound is an impurity that is found in the active ingredient, Ezetimibe Dides, which is used to treat high levels of cholesterol in the body. The fluoro impurity is a byproduct of the synthesis process of Ezetimibe Dides and is formed due to the incorporation of a fluorine atom into the molecule.
The usage of Ezetimibe Dides fluoro impurity is mainly in the pharmaceutical industry, where it is used as a reference standard in the development and quality control of Ezetimibe Dides-based products. It is an essential impurity that is monitored during the manufacturing process of Ezetimibe Dides to ensure that it is within acceptable limits.
Chemically, Ezetimibe Dides fluoro impurity is a derivative of Ezetimibe Dides, with the addition of a fluorine atom. It has a molecular weight of 409.43 g/mol, and its chemical formula is C24H22FNO3. The compound is a white to off-white powder that is soluble in organic solvents such as methanol and ethanol.
In conclusion, Ezetimibe Dides fluoro impurity is a crucial compound in the pharmaceutical industry, where it is used as a reference standard in the development and quality control of Ezetimibe Dides-based products. Its chemical formula and properties make it an essential impurity that is monitored during the manufacturing process of Ezetimibe Dides to ensure that it is within acceptable limits.
Get an Instant Quote
Related Compounds
Ezetimibe Impurity 10 | Ezetimibe ring open impurity | Ezetimibe Azetidinone Ring opened impurity | SSR-Ezetimibe | Ezetimibe O-trimethylsilyl O-benzyl Impurity | N-Nitroso Ezetimibe Impurity | Ezetimibe Impurity B | Ezetimibe Benzyl Impurity (MBZT-2) | Ezetimibe Lactone Impurity | Ezetimibe (3S,4S,3'R)-Isomer | RRR-Ezetimibe+SSS-Ezetimibe (Diastereomer mixture) | Ezetimibe Related Impurity 7 | Benzylated Ezetimibe | Methyl 5-(4-Fluorophenyl)-(5S)-hydroxypentanoate | Ezetimibe 2-Fluoro impurity | Ezetimibe Impurity 11 | Ezetimibe Lactam Cleaved Alcohol | ent-Ezetimibe | Ezetimibe Impurity 18 | m-Fluoroaniline isomer of Ezetimibe | Ezetimibe Impurity 17 | Ezetimibe Benzyl Diol Impurity | Ezetimibe Diol Impurity | Ezetimibe Diacid | Ezetimibe Impurity 25 | Benzyl Ezetimibe ether | O-Fluorobenzene isomer of Ezetimibe | Ezetimibe (3R,4R,3'R)-Isomer | Ezetimibe Impurity 1 ((3'S,3R,4S)-Desfluoro Ezetimibe) | O-Fluoroaniline isomer of Ezetimibe | Ezetimibe Impurity 15 | RSR Ezetimibe | Ezetimibe Ring-opening Dehydrate Impurity | Ezetimibe Trihydroxy Impurity | Ezetimibe Didesfluro impurity | Ezetimibe impurity (3-[5-(4-Fluoro-phenyl)-5-(R)-hydroxy-pentanoyl]-4-(S)-phenyl-oxazolidin-2-one) | Ezetimibe 2-Fluoro Hydroxy impurity | Ezetimibe Deprotected Impurity | Ezetimibe Desfluoro impurity | Ezetimibe tetrahydropyran analog | Ezetimibe Impurity C | Ezetimibe Triol Impurity |