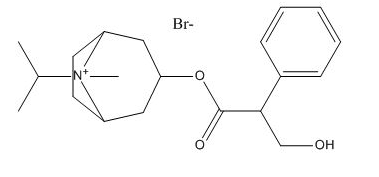
Ipratropium EP Impurity B
| Product Name | Ipratropium EP Impurity B |
|---|---|
| Alternate Names | Ipratropium Impurities, Impurities of Ipratropium |
| CAT No. | CS-T-56770 |
| CAS No. | 58073-59-9 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 412.36 g/mol |
| Mol. For. | C₂₀H₃₀BrNO₃ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Ipratropium |
| Therapeutic | Anti-Asthma / COPD |
| Smileys | CC(C)[N+]1(C2CCC1CC(C2)OC(=O)C(CO)C3=CC=CC=C3)C.[Br-] |
| Canonical Smiles | CC(C)[N+]1(C2CCC1CC(C2)OC(=O)C(CO)C3=CC=CC=C3)C.[Br-] |
| InchIKey | LHLMOSXCXGLMMN-UHFFFAOYSA-M |
| Inchl | InChI=1S/C20H30NO3.BrH/c1-14(2)21(3)16-9-10-17(21)12-18(11-16)24-20(23)19(13-22)15-7-5-4-6-8-15;/h4-8,14,16-19,22H,9-13H2,1-3H3;1H/q+1;/p-1 |
| IUPAC | (8-methyl-8-propan-2-yl-8-azoniabicyclo[3.2.1]octan-3-yl) 3-hydroxy-2-phenylpropanoate;bromide |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Ipratropium EP Impurity B is a chemical compound that is commonly used in the pharmaceutical industry as an impurity reference standard during the development of Ipratropium bromide, a medication prescribed for the treatment of chronic obstructive pulmonary disease (COPD) and asthma. Ipratropium EP Impurity B is a synthetic molecule that is not found in nature and is manufactured specifically for use as a reference standard in the quality control process of Ipratropium bromide production.
Chemically, Ipratropium EP Impurity B is a quaternary ammonium compound that contains a cyclohexane ring and a pyridine ring. This compound is a colorless to pale yellow powder that is stable under normal storage conditions. It is soluble in water and most organic solvents, and its purity is usually measured using high-performance liquid chromatography (HPLC) or gas chromatography (GC) techniques.
In the pharmaceutical industry, the accurate and precise measurement of impurities in a drug substance or drug product is essential for ensuring the safety and efficacy of the medication. Ipratropium EP Impurity B is used as a reference standard to identify and quantify impurities in Ipratropium bromide, which is critical for maintaining the quality and consistency of the medication. By using Ipratropium EP Impurity B as a reference standard, pharmaceutical manufacturers can ensure that their Ipratropium bromide products are safe, effective, and meet regulatory requirements.
Get an Instant Quote
Related Compounds
Ipratropium-d7 Bromide | Ipratropium EP Impurity C | Ipratropium Bromide Impurity A | Ipratropium Bromide Impurity 3 | Ipratropium Bromide Impurity 2 | Ipratropium EP Impurity E | Ipratropium EP Impurity D | Ipratropium Bromide impurity F | Des-Methoxy Ipratropium |