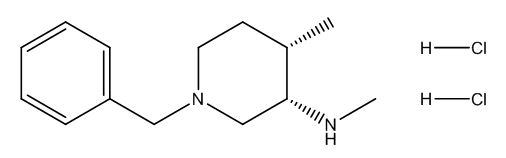
Tofacitinib impurity G
| Product Name | Tofacitinib impurity G |
|---|---|
| Alternate Names | Tofacitinib Impurities, Impurities of Tofacitinib |
| CAT No. | CS-AQ-00037 |
| CAS No. | 1354486-07-9 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 291.26 g/mol |
| Mol. For. | C₁₄H₂₄Cl₂N₂ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Tofacitinib |
| Therapeutic | Anti-Cancer / Oncology |
| Canonical Smiles | CC1CCN(CC1NC)CC2=CC=CC=C2.Cl.Cl |
| InchIKey | CVQNXCBXFOIHLH-CIKMBUIBSA-N |
| Inchl | InChI=1S/C14H22N2.2ClH/c1-12-8-9-16(11-14(12)15-2)10-13-6-4-3-5-7-13;;/h3-7,12,14-15H,8-11H2,1-2H3;2*1H/t12-,14+;;/m0../s1 |
| IUPAC | (3S,4S)-1-benzyl-N,4-dimethylpiperidin-3-amine;dihydrochloride |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Tofacitinib impurity G is a chemical compound used in the pharmaceutical industry as an impurity in the production of Tofacitinib, a medication used to treat rheumatoid arthritis and other autoimmune diseases. Tofacitinib impurity G is important because it can affect the purity, stability, and efficacy of the Tofacitinib product.
Chemically, Tofacitinib impurity G is a small, organic molecule with a molecular weight of 285.3 g/mol. Its chemical formula is C13H14N2O2, and it has a melting point of 136-138 °C.
The usage of Tofacitinib impurity G is strictly regulated in the pharmaceutical industry, and its concentration in the final Tofacitinib product must be limited to ensure the safety and efficacy of the medication. The impurity is typically removed during the manufacturing process through various purification techniques, such as chromatography and crystallization.
In conclusion, Tofacitinib impurity G is a crucial component in the production of Tofacitinib, and its careful regulation is important to ensure the purity and effectiveness of the medication.
Get an Instant Quote
Related Compounds
Tofacitinib Nitroso Impurity 4 | Tofacitinib impurity P | Tofacitinib N-Nitroso benzyl intermediate | Tofacitinib impurity Z | Tofacitinib related compound 6 | Tofacitinib S,S Isomer | Tofacitinib N-hydroxy impurity | Tofacitinib Impurity (N-Des-(2-Cyanide-acetyl)-(3S,4R)) | Tofacitinib impurity C | N-methyl-N-(4-methyl-1-nitrosopiperidin-3-yl)nitrous amide | N-Nitroso Tofacitinib Amine Impurity | Tofacitinib impurity N | N-methyl-N-(4-methylpiperidin-3-yl)nitrous amide | Tofacitinib Dihydro Impurity | Tofacitinib Nitroso Impurity | Tofacitinib Impurity 36 | N-(1-benzyl-4-methylpiperidin-3-yl)-N-methylnitrous amide | TOFACITINIB IMPURITY TOF-IV | Tofacitinib Impurity 57 | Tofacitinib impurity I | Tofacitinib impurity K | Tofacitinib Impurity 16 | Tofacitinib Diastereomer-1 and 2 | Tofacitinib Hydroxy Impurity | N-methyl-N-(4-methylpyridin-3-yl)nitrous amide | N,4-dimethyl-1-nitrosopiperidin-3-amine | Tofacitinib Nitroso Impurity 2 | Tofacitinib Impurity 29 | Tofacitinib formyl impurity | Tofacitinib Related Compound 29 | Tofacitinib N- acid Impurity | Tofacitinib impurity T | Tofacitinib Diastereomer | Tofacitinib impurity V | Tofacitinib Related Compound 26 HCl | Tofacitinib Impurity 6 | Tofacitinib Nitroso Impurity 5 | Tofacitinib N-oxide | Tofacitinib Nitroso Impurity 3 | Tofacitinib- Keto Impurity | Tofacitinib butyl ester impurity | Tofacitinib impurity J | Tofacitinib Dihydro Citricacid salt impurity | Tofacitinib Nitroso Impurity 1 |