Bisoprolol EP Impurity F
| Product Name | Bisoprolol EP Impurity F |
|---|---|
| Alternate Names | Bisoprolol Impurities, Impurities of Bisoprolol |
| CAT No. | CS-O-07378 |
| CAS No. | 1798418-82-2 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 325.4 g/mol |
| Mol. For. | C18H31NO4 |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Bisoprolol |
| Therapeutic | Anti-Hypertensives |
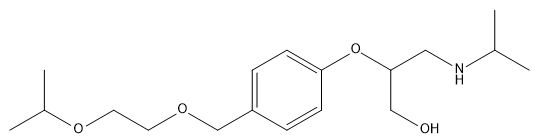
| Smileys | OCC(CNC(C)C)OC1=CC=C(COCCOC(C)C)C=C1 |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Bisoprolol EP Impurity F is a chemical compound that is used in the pharmaceutical industry as a reference standard for the quality control and analytical testing of bisoprolol, a beta-blocker medication used to treat high blood pressure, heart failure, and angina. This impurity is a degradation product of bisoprolol and is formed during the manufacturing process or storage of the drug. It is important to monitor and control the levels of impurities in pharmaceutical products to ensure their safety and efficacy.
Bisoprolol EP Impurity F is chemically known as 2-(4-{2-[(1-methylethyl)amino]ethoxy}phenyl)acetic acid. It is a white to off-white powder with a molecular weight of 303.4 g/mol. The compound has a melting point of 148-152°C and is soluble in methanol, ethanol, and water.
The chemical structure of Bisoprolol EP Impurity F contains a phenyl ring, an ethoxy group, and an amino group. The presence of these functional groups is important for the biological activity of bisoprolol and its metabolites. The impurity can be detected and quantified using analytical techniques such as high-performance liquid chromatography (HPLC) and gas chromatography-mass spectrometry (GC-MS).
In summary, Bisoprolol EP Impurity F is a degradation product of bisoprolol that is used as a reference standard for quality control and analytical testing in the pharmaceutical industry. Its chemical structure and properties are important for the safety and efficacy of bisoprolol and its metabolites.
Get an Instant Quote
Related Compounds
Bisoprolol Impurity | R-(+)-Bisoprolol Fumarate | Bisoprolol EP Impurity L hydrochloride | N-Formylbisoprolol | Bisoprolol EP Impurity B Hemifumarate (Bisoprolol n-Propyl Derivative Hemifumarate) | Bisoprolol Impurity 4 | Bisoprolol Alcohol Impurity | Dehydro Bisoprolol Hemifumarate | Bisoprolol Benzylalcohol | Bisoprolol EP Impurity B | Bisoprolol EP Impurity Q | Bisoprolol O-Phosphate | Bisoprolol Impurity 11 (mixture of isomers) | Bisoprolol glycerol diether impurity | Bisoprolol benzyl alcohol fumarate | Bisoprolol Epoxide Impurity | Bisoprolol EP Impurity M | Bisoprolol Diene Impurity | Bisoprolol Impurity 3 | Bisoprolol EP Impurity J | Bisoprolol EP Impurity N | Bisoprolol Ester Impurity | Bisoprolol EP Impurity D (TFA) | Des O-isopropyl Bisoprolol | Bisoprolol N-Methyl Impurity | Bisoprolol EP Impurity C | Dehydro Bisoprolol | Bisoprolol EP Impurity T | N-Desisopropyl-N-formyl Bisoprolol | Bisoprolol EP Impurity A | N-Acetyl Bisoprolol | Bisoprolol Carboxylic Acid Impurity | Bisoprolol EP Impurity G fumarate salt | Bisoprolol EP Impurity R | Bisoprolol Related Compound D | Bisoprolol Dimer | Bisoprolol EP Impurity F Butenedioic acid salt | Bisoprolol Phenol Impurity | Bisoprolol EP Impurity D |