Ibuprofen Impurity D
| Product Name | Ibuprofen Impurity D |
|---|---|
| Alternate Names | Ibuprofen Impurities, Impurities of Ibuprofen |
| CAT No. | CS-O-31115 |
| CAS No. | 938-94-3 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 164.20 g/mol |
| Mol. For. | C₁₀H₁₂O₂ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Ibuprofen |
| Purity | 97.6 |
| Therapeutic | Anti-Asthma / COPD |
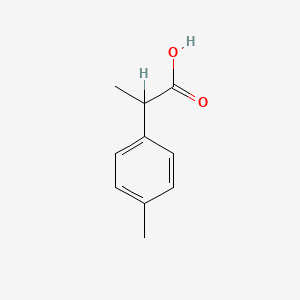
| Smileys | CC(C1=CC=C(C)C=C1)C(O)=O |
| Canonical Smiles | CC1=CC=C(C=C1)C(C)C(=O)O |
| InchIKey | KDYOFXPLHVSIHS-UHFFFAOYSA-N |
| Inchl | InChI=1S/C10H12O2/c1-7-3-5-9(6-4-7)8(2)10(11)12/h3-6,8H,1-2H3,(H,11,12) |
| IUPAC | 2-(4-methylphenyl)propanoic acid |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Ibuprofen Impurity D is a chemical compound that is commonly used in the pharmaceutical industry. It is a byproduct of the manufacturing process of ibuprofen, which is a nonsteroidal anti-inflammatory drug (NSAID) used to treat fever, pain, and inflammation. Ibuprofen Impurity D is a minor impurity that is present in small amounts in ibuprofen formulations.
The chemical name of Ibuprofen Impurity D is 2-Methyl-3-(4-isobutylphenyl) propanoic acid, and its molecular formula is C13H18O2. It is a white crystalline powder that is slightly soluble in water and has a melting point of 76-78 °C.
In terms of usage, Ibuprofen Impurity D is not intended for human consumption and is only used during the manufacturing process of ibuprofen. It is removed from the final product through purification steps to ensure that the ibuprofen formulation is safe for human use.
From a chemical perspective, Ibuprofen Impurity D is classified as an organic acid and belongs to the carboxylic acid family. It is a structurally related compound to ibuprofen and other NSAIDs, which makes it important to monitor and control its presence in the manufacturing process of ibuprofen formulations to ensure the safety and efficacy of the final product.
Get an Instant Quote
Related Compounds
Ibuprofen EP Impurity B | Ibuprofen 1,2,3-Propanetriol Esters (Mixture of Regio- and Stereoisomers) | Ibuprofen Impurity I | Ibuprofen 1,2-Propylene Glycol Esters (Mixture of Regio- and Stereoisomers) | Ibuprofen EP Impurity K | Ibuprofen Impurity H | Ibuprofen Ethyl Ester | Ibuprofen EP Impurity F | Ibuprofen impurity N | Ibuprofen 1,3-Butylene Glycol Esters (Mixture of Regio- and Stereoisomers) | Ibuprofen Methyl Ester | Ibuprofen Impurity R | Ibuprofen Ester Impurity | Ibuprofen Sorbitan Ester | Ibuprofen EP Impurity A | Ibuprofen Poly(oxy-1,2-ethanediyl) Ester | Ibuprofen impurity at RRT 1.76 | Ibuprofen USP Related Compound C | Ibuprofen 2,3-Butylene Glycol Ester | Ibuprofen impurity at RRT 1.93 | Ibuprofen EP Impurity I | Ibuprofen EP Impurity H | Ibuprofen Impurity 27 | Ibuprofen Alcohol | Tetraethyleneglycol Bisibuprofen Ester | rac a-Hydroxy Ibuprofen | Ibuprofen Impurity G | Ibuprofen impurity O | Ibuprofen Sorbitol Ester (Mixture of Diastereomers) | Ibuprofen Piconol |