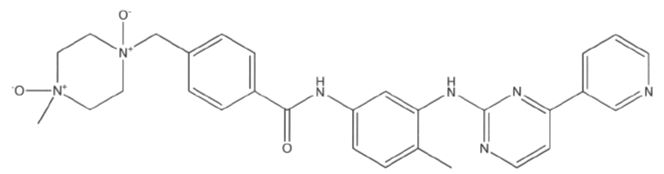
Imatinib impurity B
| Product Name | Imatinib impurity B |
|---|---|
| Alternate Names | Imatinib Impurities, Impurities of Imatinib |
| CAT No. | CS-T-56609 |
| CAS No. | 571186-93-1 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 525.6 g/mol |
| Mol. For. | C29H31N7O3 |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Imatinib |
| Purity | 95% |
| Canonical Smiles | CC1=C(C=C(C=C1)NC(=O)C2=CC=C(C=C2)C[N+]3(CC[N+](CC3)(C)[O-])[O-])NC4=NC=CC(=N4)C5=CN=CC=C5 |
| InchIKey | MFKGVMIUOYNDCV-UHFFFAOYSA-N |
| Inchl | InChI=1S/C29H31N7O3/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36(39)16-14-35(2,38)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) |
| IUPAC | 4-[(4-methyl-1,4-dioxidopiperazine-1,4-diium-1-yl)methyl]-N-[4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]phenyl]benzamide |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Imatinib Impurity B is an organic compound that is used as a reference standard in analytical laboratories to test the purity of Imatinib, which is a drug used to treat chronic myeloid leukemia and gastrointestinal stromal tumors. The chemical formula of Imatinib Impurity B is C28H32N6O3S2, and it has a molecular weight of 568.73 g/mol.
Imatinib Impurity B is a yellowish powder that is insoluble in water but soluble in organic solvents like methanol, ethanol, and acetonitrile. Its purity level is usually measured by high-performance liquid chromatography (HPLC) or gas chromatography (GC) methods.
Imatinib Impurity B is known to be an intermediate in the synthesis of Imatinib, and it is classified as a sulfoxide compound that contains a pyrimidine ring and a benzene ring. The impurity is believed to be formed during the synthesis process due to the oxidation of a thioether group.
Although Imatinib Impurity B has no therapeutic properties, it is important in the drug manufacturing process as it affects the quality, efficacy, and safety of the final product. Therefore, the accurate determination of its concentration is essential to ensure the purity and stability of Imatinib.
Get an Instant Quote
Related Compounds
Imatinib (Piperazine)-N4-Oxide | Imatinib (Pyridine)-N-oxide | Imatinib EP Impurity D (chloride) | Imatinib DiPiperazine Impurity | N-Nitroso Imatinib guanidine Intermediate | N-Nitroso Imatinib Nitro Intermediate | N-(3-methyl5-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)-4((4-methylpiperazin-1-yl)methyl)benz | Imatinib EP Impurity D | Imatinib EP Impurity C Trihydrochloride | Imatinib N-Formyl Impurity | Imatinib (N-nitroso benzamide) | N-Desmethyl -N-Nitroso Imatinib | Imatinib Impurity 3 | Imatinib Imidazole Impurity | Des-4-methylpiperazin chloro Imatinib impurity | Imatinib Impurity 5 | Imatinib N-Desmethyl Impurity | Imatinib EP Impurity C | Imatinib Impurity B | Fluorescent derivative of imatinib | Imatinib EP Impurity D Tetrahydrochloride | N-Nitroso Imatinib | Imatinib N-Acetyl Impurity | Imatinib Dimethylamino Impurity | Imatinib Impurity 16 | Imatinib Guanidino Dihydrochloride Impurity | N-Desmethyl Imatinib Mesylate | Imatinib Hydrochloride Impurity G | N-Boc-N-Desmethyl Imatinib | N-Nitroso Imatinib impurity F | N-Desmethyl -n-nitroso imatinib 1 | N-(2-Methyl-5-nitrophenyl)-4-(pyridine-3-yl)pyridine-2-amine | Imatinib Dimer |