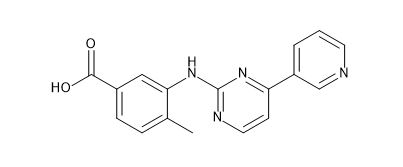
Nilotinib EP Impurity D
| Product Name | Nilotinib EP Impurity D |
|---|---|
| Alternate Names | Nilotinib Impurities, Impurities of Nilotinib |
| CAT No. | CS-EO-00346 |
| CAS No. | 641569-94-0 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 306.32 g/mol |
| Mol. For. | C₁₇H₁₄N₄O₂ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Nilotinib |
| Canonical Smiles | CC1=C(C=C(C=C1)C(=O)O)NC2=NC=CC(=N2)C3=CN=CC=C3 |
| InchIKey | LDLZPHLSVKGFSC-UHFFFAOYSA-N |
| Inchl | InChI=1S/C17H14N4O2/c1-11-4-5-12(16(22)23)9-15(11)21-17-19-8-6-14(20-17)13-3-2-7-18-10-13/h2-10H,1H3,(H,22,23)(H,19,20,21) |
| IUPAC | 4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]benzoic acid |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Nilotinib EP Impurity D is a chemical compound that is commonly used as a reference standard for the analysis and characterization of nilotinib, a medication used to treat chronic myelogenous leukemia. This impurity is known to be a major degradation product of nilotinib, and it is typically used to ensure the purity and quality of the drug during its manufacturing process.
The chemical formula of Nilotinib EP Impurity D is C21H20FN7O2, and its molecular weight is 425.43 g/mol. The compound is typically supplied as a white or off-white powder that is soluble in organic solvents such as methanol and acetonitrile.
Despite its importance as a reference standard, there is limited information available on the toxicity and safety of Nilotinib EP Impurity D. However, it is known to be a potent inhibitor of protein kinase B (AKT), which is involved in regulating cell growth and survival. This activity may be related to its potential as a degradation product of nilotinib, which also inhibits AKT.
In conclusion, Nilotinib EP Impurity D is a valuable reference standard for the characterization and analysis of nilotinib. While there is limited information available on its safety and toxicity, its potent inhibitory activity against AKT may have potential therapeutic applications in cancer treatment.
Get an Instant Quote
Related Compounds
Nilotinib Genotoxic Impurity 3 | Nilotinib Genotoxic Impurity 2 | Nilotinib Pyridine N-Oxide | Nilotinib Impurity A tetrahydrate | Nilotinib 3-Imidazolyl N-oxide | Nilotinib Chloro impurity | Nilotinib Impurity 14 | Nilotinib 5-methyl isomer | Nilotinib Impurity 11 | Nilotinib Impurity 21 | Nilotinib Impurity D | Nilotinib Nitrosamine Impurity | Nilotinib Genotoxic Impurity 1 | Nilotinib Impurity 3 | Nilotinib Impurity A | Nilotinib Amine impurity | Desmethyl nilotinib | Nilotinib Impurity 17 | Nilotinib 2-methyl isomer |